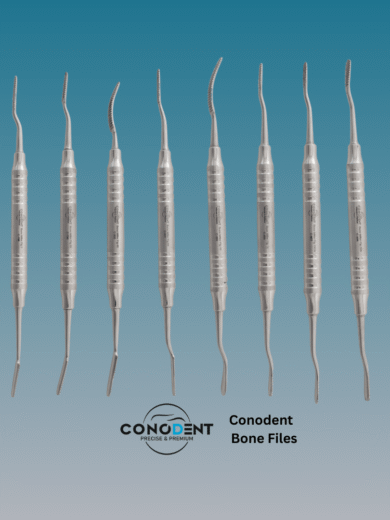
CONODENT Miller Bone File – Hollow Handle (CDT-C-800)
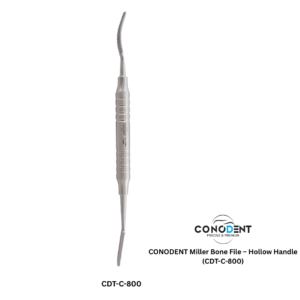
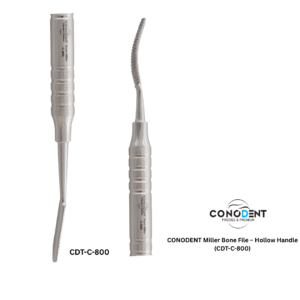
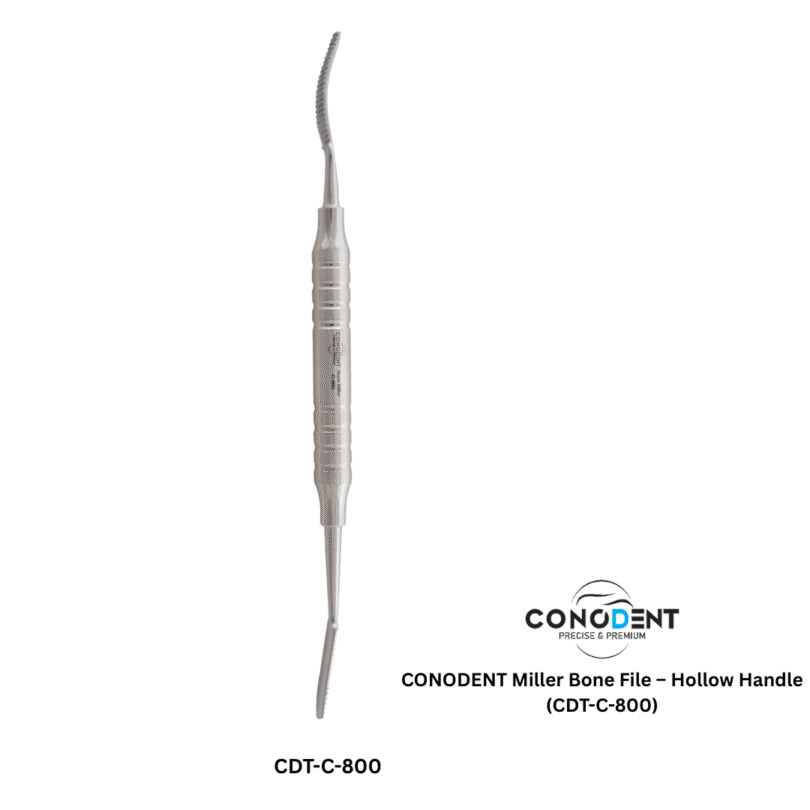
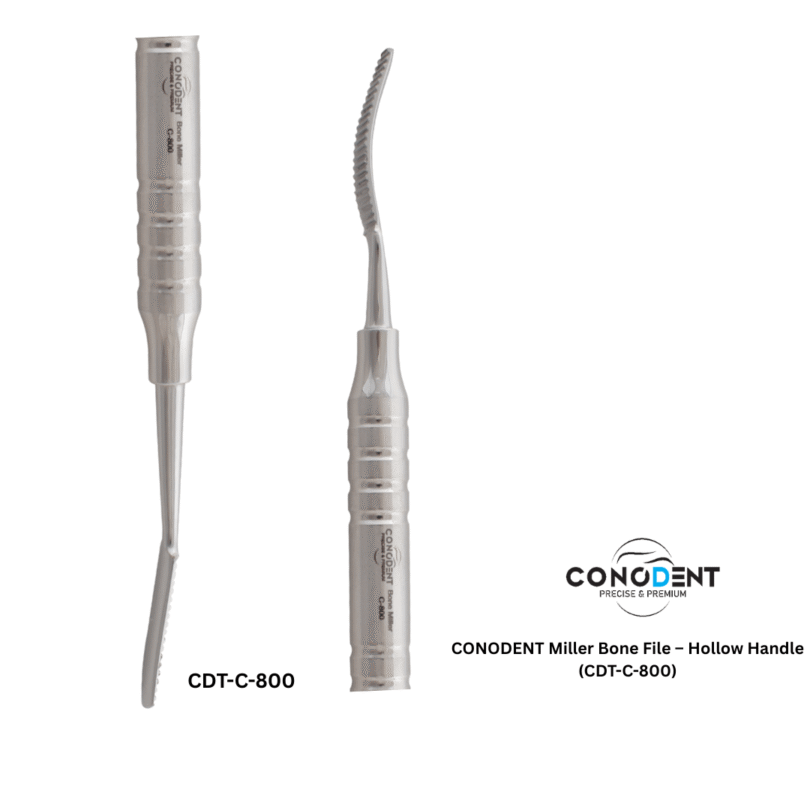
The CONODENT Miller Bone File (Product Code: CDT-C-800) is a premium surgical instrument designed for precision bone contouring and smoothing during oral and maxillofacial procedures. Featuring a lightweight, ergonomic hollow handle, this instrument provides superior tactile feedback and reduces hand fatigue during extended surgical use.
Key Features and Specifications
Instrument Name: Miller Bone File
Product Code: CDT-C-800
Handle Type: Ergonomic Hollow Handle for enhanced grip and comfort.
Material: Crafted from high-quality, imported medical-grade stainless steel, ensuring exceptional durability and resistance to corrosion.
Precision Performance: Designed with specialized serrations for efficient smoothing of bone fragments and narrow areas.
Quality Assurance & Manufacturing
CONODENT instruments represent a commitment to excellence in surgical manufacturing. Our products are produced by Medical Supplier in Oman, adhering to the highest global industry benchmarks:
Standards Compliance: Manufactured in strict accordance with GCC and European standards.
Certified Facility: Produced in an ISO 13485:2016 certified facility, ensuring a rigorous quality management system for medical devices.
Sterilization: Fully autoclavable and designed to withstand repeated sterilization cycles without compromising structural integrity.
Technical Specifications: CDT-C-800
| Feature | Specification Details |
| Product Name | CONODENT Miller Bone File |
| Product Code | CDT-C-800 |
| Instrument Type | Bone Shaping & Smoothing File (Miller Type) |
| Handle Design | Light weight Ergonomic Hollow Handle |
| Handle Diameter | Approximately 8mm to 10mm (Tactile Grip) |
| Working Ends | Double-Ended (Straight & Curved Ends) |
| Serration Type | Precision Straight-Cut (Parallel Serrations) |
| Material | High-Quality Imported Medical Grade Stainless Steel |
| Total Length | Approximately 180mm (18cm / 7″) |
| Manufacturing Origin | Medical Supplier, Oman |
| Quality Certification | ISO 13485:2016 |
| Compliance Standards | GCC and European (CE) Standards |
| Sterilization | Fully Autoclavable / Reusable |
| Usage | Oral, Periodontal, and Maxillofacial Surgery |





There are no reviews yet.